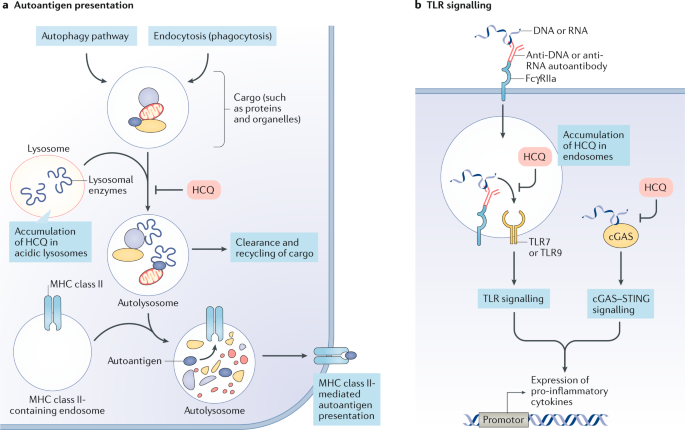
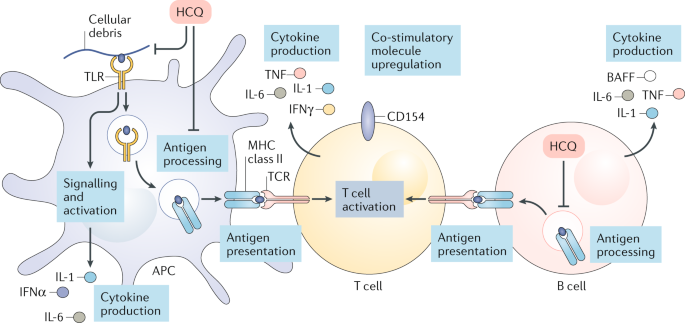
It has been a few weeks since our last post on the blog. It seems that COVID-19 has shown us it is here to stay for the time being. Local legislators, public health officials, and politicians are trying to navigate how to safely return to a somewhat normal life while keeping infection rates low. One of the biggest questions that has come up is whether or not we can safely return children to schools without risking a further spread of COVID-19.
While some schools have reopened and faced infection among their staff and students, other areas are still deciding whether students will stay remote or return to school in a hybrid learning format. A few weeks ago a photo from a Georgia High School was brought to the attention of the media. The video showed crowded hallways and many high schoolers not wearing a mask. It has been reported that in this school, there have been a handful of positive cases reported. Schools in Mississippi, Tennessee, Indiana, and North Carolina have all reportedly opened and noted positive cases causing the schools to close or students to quarantine. (Read the New York Mag article here.)
Worldwide…
According to the John Hopkins’ Dashboard there are now more than 20 million cases worldwide. Based on a 5 day average of new cases, numbers are rising in India, Colombia, and Peru. There have been over 780,000 deaths globally.

In the United States…
As of Wednesday afternoon, there have been 5,460,429 positive cases. According to the CDC website, there were about 40,000 new cases reported in the last 24 hours. This number has decreased over the last few days, along with the number of positive testing samples. The national average of tests that were positive decreased from 8.7% (reported by the CDC on July 25th) to 7.0% as of the data reported by the CDC on the week ending August 8th. As of August 8th, the South East (11.5%) and South Central (12.6%) regions continued to have the highest percentages of specimens testing positive for SARS-CoV-2. If you look closely at the chart below, you can see the rise in the grey and red lines, which represent the pediatric population and percent positivity of tests.

In Children…
The AAP released its data on Children and COVID-19 for the last three weeks. What was notable from this data reported was that children accounted for about 9% of all COVID cases in the US for a total of 406,109 total child COVID-19 cases as of the data on 8/13. What was most alarming was the 40% increase in COVID cases from 7/16-7/30 for a total of 97,078 new child cases reported. From 7/9-8/6 there were 179,990 new cases reported in children (200,184 to 380,174) which was a 90% increase over that 4 week period. Despite this increase, children were only reported as 0.5-4.6% of all COVID hospitalizations and 0-0.4% of all COVID deaths.

So we know that disease is less severe in children, however, about 1 month following COVID-19 exposure was the initial presentation of MIS-C which we summarized here & here. So although symptoms of COVID-19 may not be as severe, we have yet to see the spike in MIS-C cases following this rapid increase in pediatric COVID-19 cases.
I am not sure about my other colleagues in pediatrics, but I have had many friends ask about safely sending their children back to school. It is a difficult question to answer as I am not a parent who has to balance child care needs with my work schedule. This question is one that is even stumping many experts in the field. The AAP released their updated guidance on the matter on Wednesday.
We may not have the best answer to this question, but we do have some data that can help guide our management. Earlier studies may have given us false reassurance that children were not as affected by COVID-19, however more recent studies have discussed transmission of SARS-CoV-2 among pediatric patients and demonstrated this may not be the case.
Up first, South Korea’s Contact-Tracing Program…
An early release article titled “Contact Tracing during Coronavirus Disease Outbreak, South Korea, 2020” in Emerging Infectious Diseases described the data collected from the robust contact tracing program that was utilized in South Korea during the pandemic.1 The program identified 59,073 contacts of 5,706 index patients. (Index patients were defined as the first laboratory confirmed case or first documented case in a cluster.) They then grouped index patients by age and noted how many positive cases came from these index patients. A “case” was defined as a contact with symptom onset after that of a confirmed COVID-19 index patient. Patients were monitored for an average of 9.9 days after infection was confirmed.
- Overall, a total of 11.8% of household contacts of index patients had COVID-19 (95% CI 11.2%-12.4%)
- For children age 0-9, there were 29 index patients and 237 contacts were traced, while for children age 10-19 there were 124 index patients and 457 contacts were traced. These numbers were lower than the remaining age groups.
- In household contacts of patients age 10-19 years of age, 18.6% of contacts had COVID-19 (95% CI 14.0%-24.0%)
- For most age groups COVID-19 detection was significantly higher in household contacts than non household contacts.
- Children aged 0-9 had a lower number of positive household contacts- reported at 3 positive of 57 contacts, for 5.3% positive with a 95% CI (1.3-13.7).
So what does this tell us? Children aged 10-19 had the highest positivity rate among their household contacts, while non household contacts for children ages 0-9 years old had positivity slightly lower than the entire contact tracing program. However, the authors remind us in their conclusions that this data was collected in the middle of school closures, so the detection rates may be altered, but this information should most definitely be considered as schools begin to reopen.
On to Switzerland and COVID-19 in Families…
A brief released titled “COVID-19 in Children and the Dynamics of Infection in Families” in Pediatrics described patients under 16 years old with SARS-CoV-2 infection from March 10 – April 10, 2020 in Geneva University Hospital’s Surveillance Network.2 This study identified a total of 4310 patients with SARS-CoV-2 Infection, 40 patients (0.9%) were under 16 years old.
- The median age of the study patients was 11.1 years old
- The most commonly reported symptoms for the cohort were cough, fever, nasal discharge, and headache.
- The study evaluated familial clusters and looked at 111 household contacts (HHCs) of the study children
- 39 mothers, 32 fathers, 23 pediatric siblings, 8 adult siblings, and 7 grandparents were evaluated.
- In the 39 study cases, 31 Adult HHCs (79%) were suspected or confirmed with COVID-19 prior to the study child suggesting that children may be mainly infected in familial clusters
- Children developed symptoms first in only 8% of households (3/39)
- In the households, adult HHCs developed symptoms about 85% of the time vs only 43% of pediatric HHCs (p< .001)
This study was similar with previous data that showed children as index cases in <10% of familial clusters of SARS-CoV-2.

et al. COVID-19 in Children and the Dynamics of
Infection in Families. Pediatrics. 2020;146(2):
e20201576
Next, age related NP SARS-CoV-2 Levels in Chicago…
A Research Letter titled “Age-Related Differences in Nasopharyngeal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Levels in Patients With Mild to Moderate Coronavirus Disease 2019 (COVID-19)” was published in JAMA Pediatrics on July 30th.3 This letter presented the data collected between March 23 and April 27, 2020 on SARS-CoV-2 reverse transcriptase-polymerase chain reaction (PCR) swabs in individuals ages 1 month to 65 years that presented to various testing centers in Chicago, IL. This letter described the PCR amplification cycle threshold (CT) of the samples collected and analyzed. Click on the link for more detailed information but a CT is the number of cycles it takes for a signal to cross the fluorescent threshold, and this has been shown to be inversely proportional to the amount of target nucleic acid in the sample, ie less cycle thresholds = more nucleic acid). It had been previously reported that patients with severe infection had lower CT values, so 7 children who required supplemental oxygen were excluded from this analysis. In addition 7 asymptomatic patients, 29 patients with unknown duration of symptoms, and 19 patients with symptom onset one week prior to collection were excluded.
- The final cohort for analysis included 145 patients grouped by age: <5 years old (n=46), 5 to 17 years old (n=51), and 18 years to 65 years (n=48)
- The median CT value for ages 5-17 was 11.1 [interquartile range 6.3-15.7] and the median CT value for ages 18-65 was 11.0 [IQR 6.9-17.5]
- For children under 5 years old there was a significantly lower median CT value from the other two groups at 6.5 [IQR 4.8-12.0].
- This indicates that children have equivalent or more viral nucleic acid in their upper respiratory tract compared to older children and adults.
- These observed values demonstrate an approximate 10-fold to 100-fold greater amount of SARS-CoV-2 in the upper respiratory tract of young children
- When including the patients with unknown duration of symptoms the statistical difference between the groups was similar.

So what does all this data tell us?….
We know from previous data that children overall have less severe disease and lower hospitalization and mortality rates due to COVID-19. What we can see from the limited data presented here is that although less likely to be the first infected case in the household children are still likely to spread SARS-CoV-2 infections to their household contacts, especially those aged 10-19. The data also shows that there may be more SARS-CoV-2 in the upper respiratory tracts of younger children under 5 years old. So school aged children are capable of spreading SARS-CoV-2 based on what we see here. As we begin to see schools open here in the United States, we will have more data on our pediatric cases and transmission. For now, the jury still remains out on the safest approach for Schools in the setting of SARS-CoV-2.
Here are a few more recommended readings:
P.S…. In our high schoolers we already know vaping is bad. Mix vaping and COVID-19 infections? Even worse. https://www.cnn.com/2020/07/13/health/young-adults-smoking-risk-coronavirus-wellness/index.html
Thanks for reading along and stay safe! As always, you can contact us here.
Resources:
- Park YJ, Choe YJ, Park O, Park SY, Kim YM, Kim J, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020 Oct [date cited]. https://doi.org/10.3201/eid2610.201315
- Posfay-Barbe KM, Wagner N, Gauthey M et al. COVID-19 in Children and the Dynamics of Infection in Families. Pediatrics. 2020;146(2): e20201576
- Heald-Sargent T, Muller WJ, Zheng X, Rippe J, Patel AB, Kociolek LK. Age-Related Differences in Nasopharyngeal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Levels in Patients With Mild to Moderate Coronavirus Disease 2019 (COVID-19). JAMA Pediatr. Published online July 30, 2020. doi:10.1001/jamapediatrics.2020.3651